July 1, 2024
Radionetics provides Lilly access to proprietary GPCR targeting small molecule radiopharmaceuticals. Radionetics receives $140 million upfront payment and Lilly obtains the exclusive right to acquire Radionetics for $1 billion.
Part of a treatment strategy known as theranostics, radiopharmaceuticals can be used for both therapy and diagnosis.
Radiopharmaceuticals, when tethering a radionuclide suitable for imaging, create a map of cancerous tumors in the body that provides precise diagnostic information. From the imaging results, the patients who are most likely to respond are identified and treated with individualized regiments of the therapeutic version of the same radiopharmaceutical. This precision medicine approach seeks to make substantial improvements in clinical outcomes.

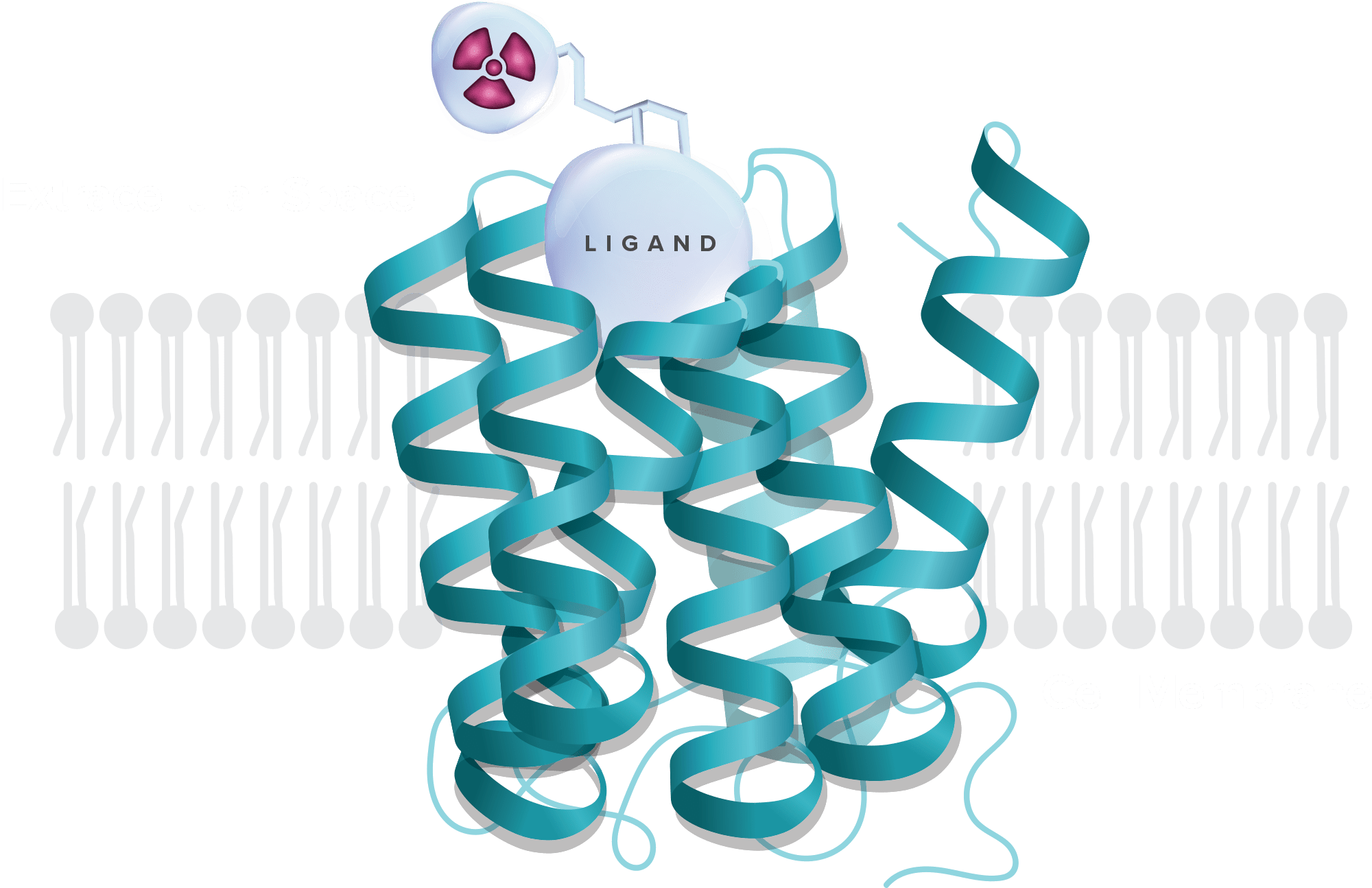
Small molecules, tethered with powerful radioisotopes, are designed to exquisitely bind to receptors overexpressed in cancer and deliver powerful radiation to kill tumors, without affecting normal tissue.
Our unique approach marries a vast knowledge of GPCR biology with unrivaled proficiency in small molecule medicinal chemistry to develop potent and highly selective radiopharmaceuticals. We take a fit-for-purpose approach, pinpointing the precise target and selecting the ideal small molecule ligand paired with the optimal radioisotope to achieve a therapeutic effect.
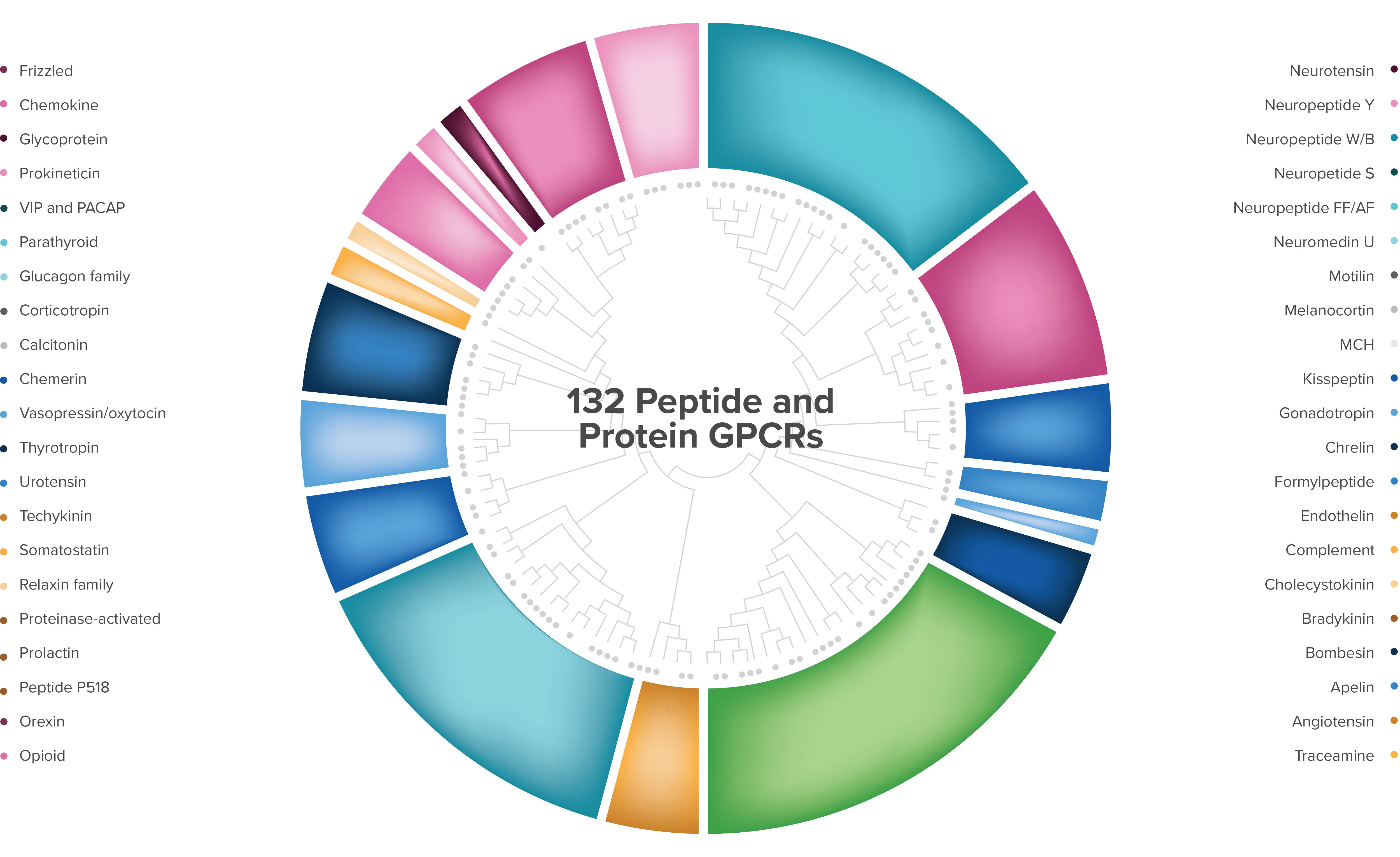
Largest gene family in humans
132 known protein/receptor pairs
>65 with increased tumor expression
Radionetics is advancing a pipeline of novel small molecule radioligands targeting GPCRs for the treatment of a broad range of cancers, including breast cancer, lung cancer, and other indications of high unmet need.












Our team is highly collaborative and dynamic, and we are doing exciting and novel research driving radiotherapeutics into the clinic and towards patients.
Radionetics is headquartered in San Diego, California, in the heart of the thriving life sciences community. Our team includes talented individuals who are experts in their respective fields and united by a shared passion for improving the lives of cancer patients.
Radionetics has a competitive total compensation package that includes bonus opportunity; equity; medical, dental, vision, life/AD&D, short-term, and long-term disability insurance; 401(k) retirement plan; 4 weeks of paid time off annually; and generous paid holidays.
We invite you to review our open positions and submit your application if there is a suitable match with your background and experience.

Umesh Gangadharmath, Ph.D. is Chief Technical Officer with more than 19 years of experience in radiopharmaceutical development and GMP manufacturing across academic and commercial settings. His expertise spans oncology, cardiology, and neurology, with a focus on PET imaging agents and radioligand therapies.
Dr. Gangadharmath has led radiopharmaceutical programs from preclinical development and IND-enabling CMC through early clinical research and full clinical production and has established GMP manufacturing facilities globally to support clinical trials. He was a founding member of Optimal Tracers, a radiopharmaceutical CDMO, where he spent a decade building the organization from concept to a fully operational GMP facility, later acquired by Telix Pharmaceuticals. His team also contributed to the establishment of a GMP radiopharmaceutical manufacturing facility at University Hospitals Cleveland Medical Center.
Previously, Dr. Gangadharmath served as Director of the biomedical cyclotron facility at UCLA and as a Senior Scientist in Radiochemistry at Siemens Molecular Imaging Biomarker Research, where he was part of the team that developed TAUVID® (flortaucipir). He has served on the USP Radioactive Drugs Expert Panel and established a molecular imaging division at Loma Linda University Medical Center.
Dr. Gangadharmath holds an M.Sc. and Ph.D. in Inorganic Chemistry from Karnatak University, India, and completed postdoctoral training at the University of California, Los Angeles.